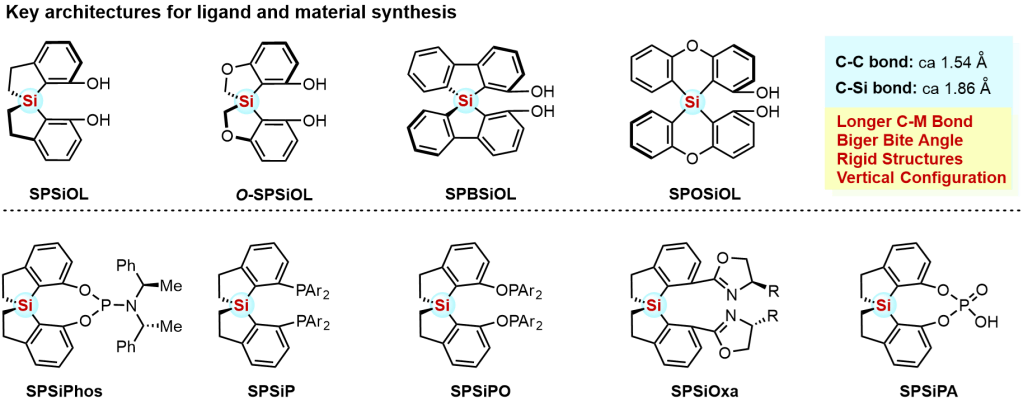
The development of privileged chiral architectures which are versatile for various chiral ligands or organocatalysts preparation with superior performance in a variety of organic transformations has significant pushed forward the advancement of asymmetric catalysis. However, only a few scaffolds could be seen as privileged chiral backbone, including BINOL-, SPINOL-, TADDOL-based architectures. In the Wang lab, our project mainly aims at the design of the novel heteroatom (Si, Ge etc.)-centered spirocyclic ligand skeletons, developing new asymmetric methods for construction of those heteroatom-centered spirocyclic skeletons, preparation of various types of heteroatom-centered spirocycle-based ligands and catalysts, and further studying their structure-function relationships in organic synthesis.
For representative reports, see:
(a) Chang, X.; Ma, P.-L.; Chen, H.-C.; Li, C.-Y.*; Wang, P.* Asymmetric Synthesis and Application of Chiral Spirosilabiindanes. Angew. Chem. Int. Ed. 2020, 59, 8937–8940.
(b) Yang, L.; Xu, W.-Q.; Liu, T.; Wu, Y.; Wang, B.-Q.*; Wang, P.* Concise Synthesis and Application of Enantiopure Spirobiphenoxasilin-Diol and Its Related Chiral Ligands. Chem. Commun. 2021, 57, 13365–13368.
(c) Liu, T.; Mao, X.-R.; Song, S. Chen, Z.-Y.; Wu, Y.; Xu, L.-P.*; Wang, P.* Enantioselective Nickel-Catalyzed Hydrosilylation of 1,1- Disubstituted Allenes. Angew. Chem. Int. Ed. 2023, 62, e202216878.
(d) Li, H.; Zhao, P.-G.; Wang, C.-Y.; Zhang, R.-Y.; Li, J.-J.; Wu, Y.; Wang, P.* SPSiPs, A Class of Diphosphine Ligands Based on SPSiOL with a Large Dihedral Angle. Org. Lett. 2023, 25, 3859–3863.
(e) Li, Z.-D.; Ren, F.; Wu, Y.; Li, J.-J.; Luo, J.*; Wang, P.* Development and Application of SPOSiPs: A Class of Diphosphine Ligands Based on SPOSiOL. Org. Lett. 2024, 26, 7436–7441.
(f) Chen, S.-H.; Zhang, S.; Chen, Z.-Y.; Wu, Y.; Wang, P.* Cu-Catalyzed Enantioselective Carbene Insertion into Ge–H and Si–H Bonds Enabled by SPSiBox with a Tunable Chiral Pocket. J. Am. Chem. Soc. 2025, 147, 15666–15675.